Online Marine Electrical Courses Basic Theory
All online marine electrical courses basic theory is the start point. This is the first Module 1 in the Online Marine Electrical course. It is intended to cover basic theory and maintain a practical focus and be learnt in context. There are several basic and fundamental theories to understand, along with the history element. These will be covered without delving too deep into the more complex theories and mathematical formulae required for professional training.
Much of what is covered in the basic electrical theory will be required to increase understanding in the following modules. Understanding basic electrical theory will allow course participants to carry out virtually all of the installation, testing and troubleshooting on most marine electrical and electronic installations on your own boat and perhaps assist another boat owner. Online marine electrical courses basic theory is the start point.
Electricity – A Basic History
Electricity has a history dating back over 2500 years, when the Greeks discovered the effects created by rubbing amber with other materials. The amber became statically charged and attracted various materials such as feathers, cloth and dry leaves. The Greeks called amber “electron” and the term electric was derived from this original word. It means “to be like amber” or have the power to attract other objects or materials. It took another 2000 years until this phenomenon was further explored with various experiments and research. William Gilbert discovered in the early 1600’s that amber was not the only material that could be charged or retain a charge. Gilbert called materials that can be charged “electriks” and those that could not be charged “noelectriks”. In 1773, the Frenchman, Charles DuFay discovered that charged glass could also repel some charged objects and attract others and that the force of repulsion was as important as the force of attraction. Online marine electrical courses basic theory is the start point.
Electricity – A Basic History
These experiments resulted in the formation of two lists.
1. List A. This included glass rubbed on silk; glass rubbed on wool or cotton; mica rubbed on cloth; asbestos rubbed on cloth or paper and a stick of sealing wax rubbed on wool.
2. List B. This included hard rubber rubbed on wool; a block of sulfur rubbed on wool or fur; most types of rubber rubbed on cloth; sealing wax rubbed on silk, wool or fur, glass or mica rubbed on dry wool, and amber rubbed on cloth.
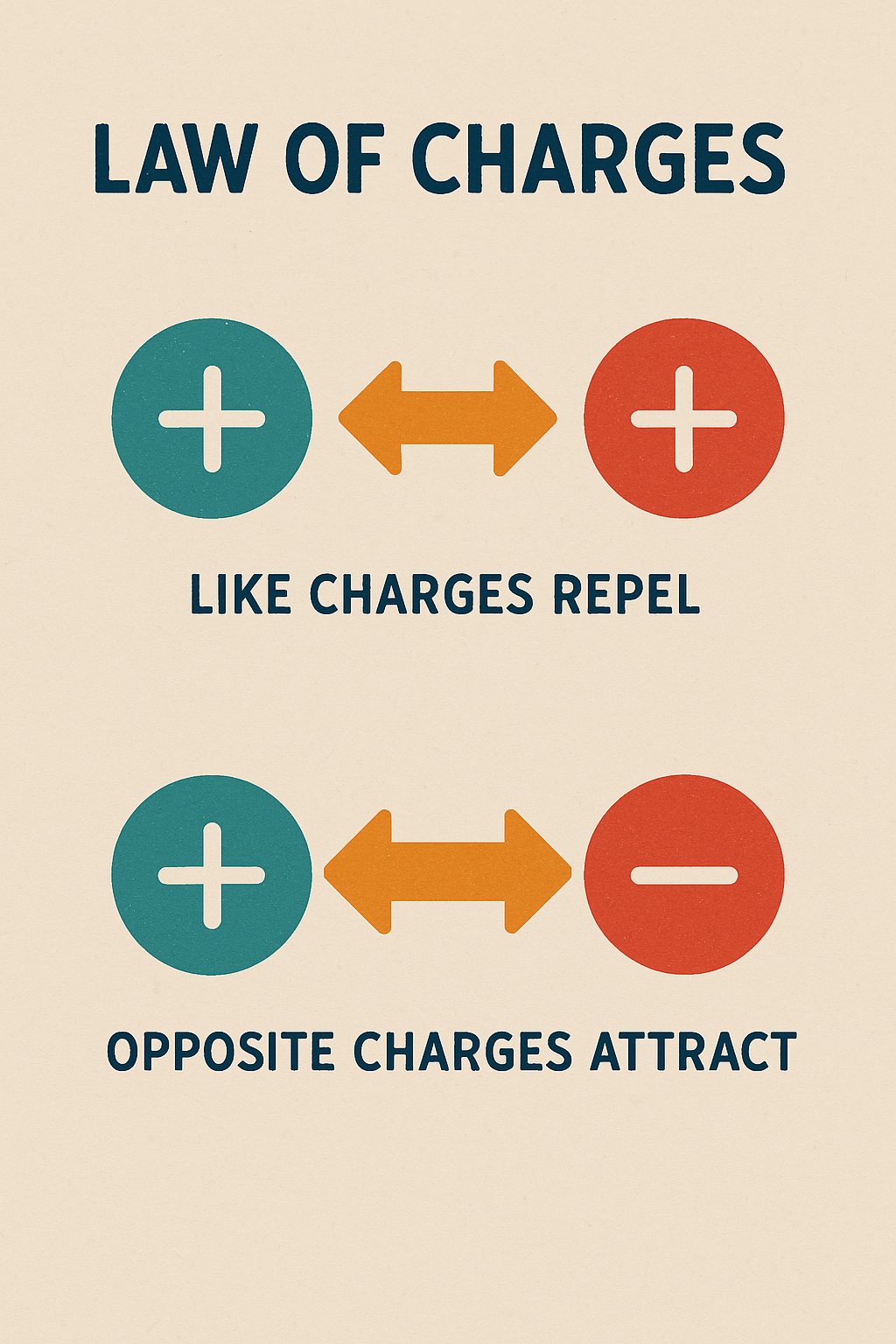
The materials in List A attract any material in List B. All the materials in List A will repel each other. All of the materials in List B will repel each other. It was Benjamin Franklin who named the materials in List A positive and the materials in List B negative. The first material in each list was used as a standard for determining whether the charged object is either positive or negative. Any object repelled by a piece of glass rubbed on silk has a positive charge and any item repelled by a hard rubber rod rubbed on wool will have a negative charge.
The
Law of Charges
The Law of Charges states that opposite charges attract and like charges repel. This means that two objects with an opposite charge will be attracted to each other. The two positively charged objects and two negatively charged units will repel each other. This is because lines of force can never cross each other. The outward-going lines of force of a positively charged object combine with the inward-going lines of force of a negatively charged object. This produces an attraction between the two objects. If the two objects with like charges come close to each other, the lines of force will repel. As the nucleus has a net positive charge, and electron a negative charge, the electron is attracted to the nucleus. The law of charges, also known as Coulomb’s Law, and it is used within various everyday situations, some real world examples are as follows:
1. Photocopiers and Laser Printers: These devices use electrostatic charges to transfer toner onto paper, demonstrating Coulomb’s Law in action.
2. Powder Coating: In industrial applications, charged powder particles adhere to surfaces due to electrostatic forces.
3. Electrostatic Air Cleansing: Air purifiers use charged plates to attract dust and pollutants, helping to clean the air.
Basic Atomic Theory
The atom is the basic
building block of everything within the universe and all matter comprises a
combination of atoms. Matter is defined as any substance that has mass and
occupies space. Matter exists as a solid, liquid, or a gas. An element is a
substance that cannot be chemically divided into a simpler substance. An atom
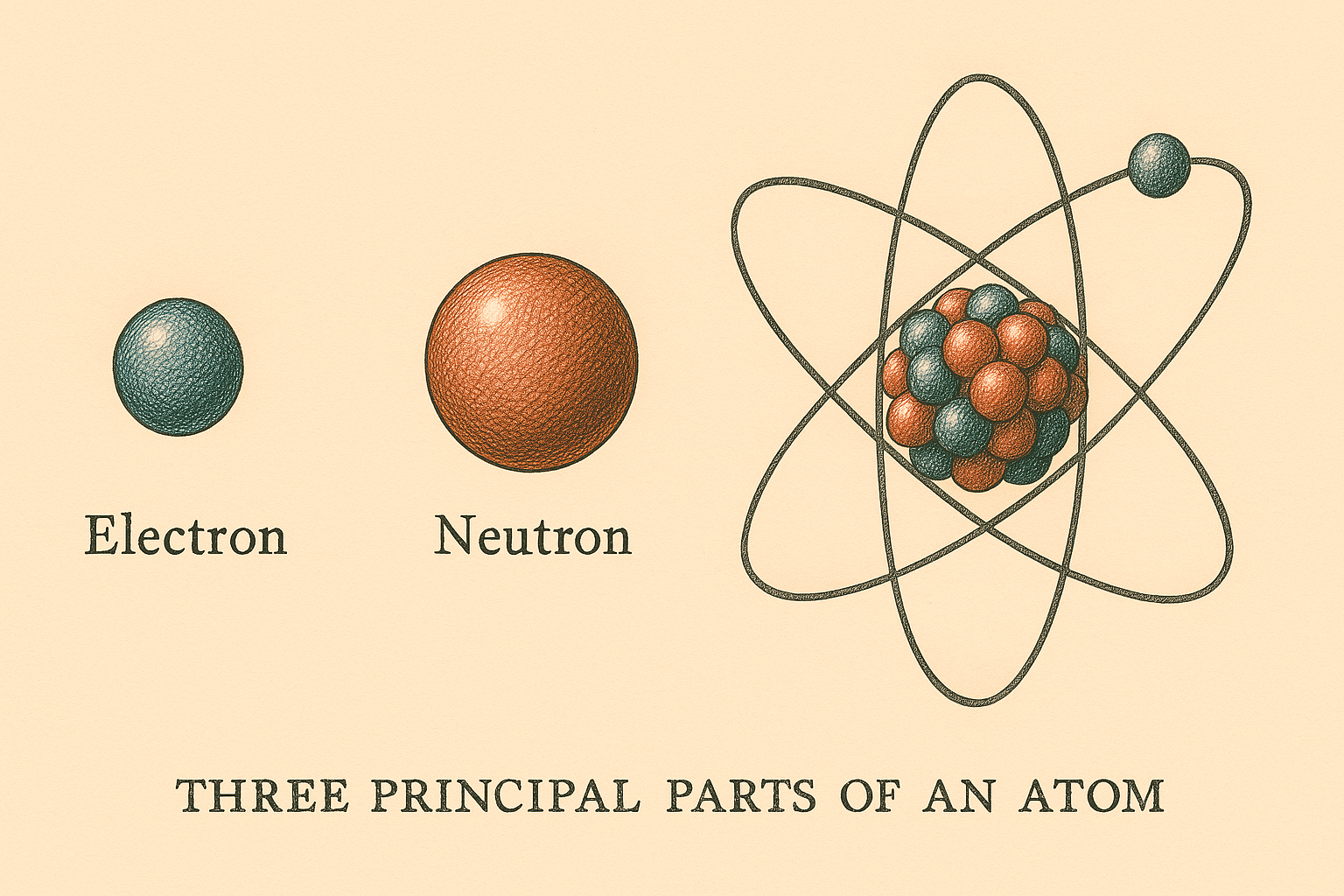
is the smallest part of an element. The three principal parts of an atom are
the electron; the neutron, and the proton. It is theorized that protons and
neutrons are actually made of the smaller particles called quarks. Online marine electrical courses basic theory is essential to understand.
The proton has a positive charge, the electron has a negative charge, and the
neutron has no charge. The neutron and proton combine to form the nucleus of
the atom. Since the neutron has no charge, the nucleus will have a net positive
charge. The number of protons in the nucleus determines what kind of element an
atom is. Oxygen, for example, contains 8 protons in its nucleus, and gold
contain 79. The atomic number of an element is the same as the number of
protons in the nucleus. The lines of force produced by the positive charge of
the proton extend outward in all directions. The nucleus may or may not contain
as many neutrons as protons. For example, an atom of helium contains two
protons and two neutrons in its nucleus, while an atom of copper contains 29
protons and 35 neutrons.
The electron orbits around the outside of the nucleus. An electron is about
three times as large as a proton. The estimated size of a proton is 0.07
trillionth of an inch in diameter, and the estimated size of a proton is 0.22
trillionth of an inch in diameter. Although the electron is larger in size, the
proton weighs about 1840 times more.
Online Marine Electrical Courses Basic Theory - Atomic Theory
Elements differ based on their atomic structure in several fundamental ways:
- Atomic Number – Each element has a unique number of protons in its nucleus, known as its atomic number. This determines the element’s identity. For example, hydrogen has one proton, while oxygen has eight.
- Electron Configuration – The arrangement of electrons around the nucleus varies, influencing an element’s chemical properties and reactivity. Elements with similar electron configurations belong to the same group in the periodic table.
- Neutron Count and Isotopes – While an element always has the same number of protons, it can have varying numbers of neutrons, leading to different isotopes. For instance, carbon-12 and carbon-14 both have six protons, but carbon-14 has two extra neutrons.
- Atomic Mass – The total number of protons and neutrons determines the atomic mass. Heavier elements generally have more protons and neutrons than lighter ones.
Chemical Properties – The way elements bond and react with others depends on their electron configuration, especially their valence electrons. For example, noble gases are mostly non-reactive due to their full outer electron shells
What are Valence Electrons?
The outer shell of an
atom is called the valence shell. Any electrons located in this outer shell of
an atom are known as valence electrons. The valence shell of an atom cannot
hold more than eight electrons. It is the valence electrons that are important
in the understanding of how electricity works. A conductor is made from a
material that contains one or two valence electrons. Atoms with one or two
valence electrons are unstable and can be made to give up or lose these
electrons with minimal effort. Conductors are materials that permit electrons
to flow through them easily. When an atom has only one or two valence
electrons, these electrons are loosely held by the atom and are easily given up
for the current flow. Silver, copper, gold, and aluminum all contain one
valence electron and are excellent conductors of electricity. Silver is the
best natural conductor of electricity, followed by copper, gold, and aluminum. Online marine electrical courses basic theory is the start point. Get your copy of the Marine Electrical and Electronics Bible r4th Edition here.
What
is Electron Flow?
Electrical current is the flow of electrons. It is produced when an electron from one atom knocks electrons of another atom out of orbit. When an atom contains only one valence electron, that electron is easily given up when struck by another electron. The striking electron gives its energy to the electron being struck. The striking electron settles into orbit around the atom, and the electron that was struck moves off to strike another electron. Some energy is also lost when one electron strikes another. That is what causes the heat in a wire when current flows through it. If too much current flows through a wire, overheating will damage the wire. If an atom containing two valence electrons is struck by a moving electron, the energy of the striking electron will be divided between the two valence electrons. If the valence electrons are knocked out of orbit, they will contain only half the energy of the striking electron. Material containing seven or eight valence electrons are known as insulators. Insulators are materials that resist the flow of electricity. When the valence shell of an atom is full, the electrons are held tightly and are not given up easily. Examples of insulator materials are rubber, plastic, glass, and wood. The energy of the moving electron is divided so many times that it has little effect on the atom. Any atom that has seven or eight valence electrons is extremely stable and does not easily give up an electron. Electrons can be freed from their orbit by applying an external force, such as movement through a magnetic field, heat, friction, or a chemical reaction. Online marine electrical courses basic theory is the start point.
What is Electron Flow?
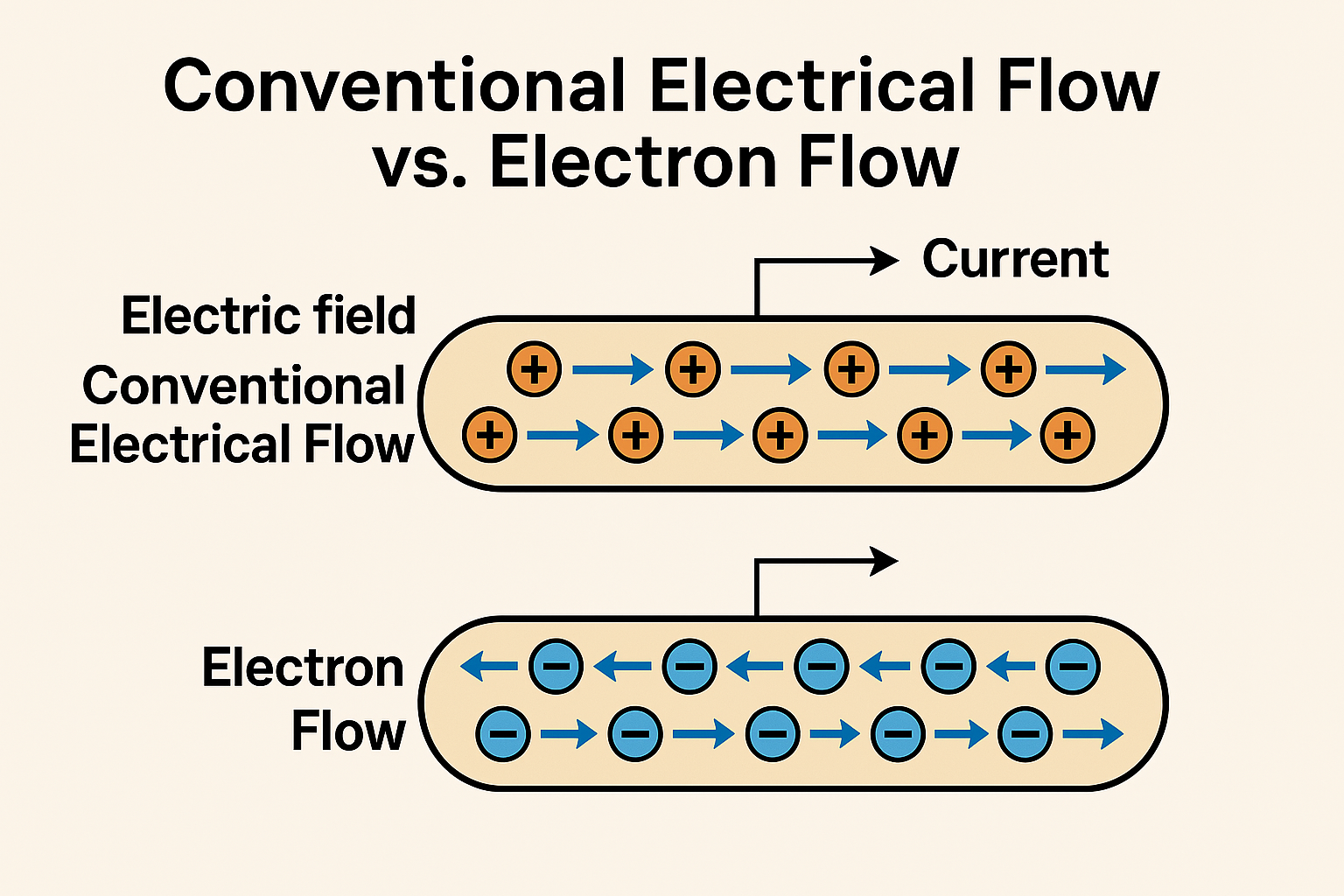
Conventional Current Flow (positive to negative)
1. Circuit diagrams and engineering designs. Most circuit schematics, whether for microcontrollers or simple light switches, follow conventional current flow.
2. Basic Electrical Circuits: Used in household wiring, where current is described as flowing from the power source's positive terminal to the negative terminal.
3. Helps engineers and electricians predict circuit behavior.
4. Common in electrical textbooks and industry standards.
Electron Flow (negative to positive)
1. Physically accurate representation of actual electron movement.
2. Semiconductor Devices: In transistors and diodes, electron flow is crucial for understanding behavior at the atomic level, especially in PN junctions.
3. Batteries: Battery chemistry relies on electron movement, such as in lithium-ion cells where electrons flow from the negative terminal to power connected devices.
Sources of Electricity
There are six primary methods for producing a voltage or electromotive force:
1. Friction.
Voltage is produced by rubbing certain materials together.
2. Pressure (piezoelectricity). Voltage is produced by squeezing crystals of certain substances.
3. Heat (thermoelectricity). Voltage is produced by heating the joint (junction) where two unlike metals are joined.
4. Light (photoelectricity). Voltage is produced by light striking photosensitive (light sensitive) substances which for boats is the solar panel cell.
5. Chemical action. Voltage is produced by a chemical reaction within a battery cell, that includes flooded cell lead acid, Lithium-ion and alkaline cells.
6. Magnetism. Voltage is produced in a conductor when the conductor moves through a magnetic field, or a magnetic field moves through the conductor so that the magnetic lines of force of the field are cut. This applies to engine starter motors and other electric motors that power your pumps and alternators along with wind and water generators.
Module Summary and Quiz
So how did you do on those basics of electricity. It should help with some understanding and I have added some quizzes to self test. Online marine electrical courses basic theory is the start point.