Marine Electrical Courses Online
Batteries
The Marine Electrical Courses Online Batteries subject is a major one for most boat owners. The demands that are now placed on the average boat electrical system have generally outgrown that of battery technology with the rapid advances in electrical and electronics systems. The relatively lower consumption equipment such as instrumentation and traditional high demand equipment such as autopilots and radar have now been eclipsed. The new power thirsty gear list is topped by the AC inverter, which extracts a heavy price in electrical power for the comforts it delivers. That is where Lithium ion batteries have progressed, with much higher power density, much lower weights and more.
The bottom line on power calculations has risen significantly, and consequentially so has the battery power required to support it. The search for a bullet proof and fault tolerant battery bank is as real as ever. The flooded cell lead acid battery has always filled the role and it's position is under siege from technology newcomers, with comparison testing being performed under various controlled test conditions between all the battery types. The performance claims and counterclaims are often confusing as are the various numbers are worked out, and each successive innovation claims greater cycle abilities and life expectancies than previous. Marine Electrical Courses Online Batteries is all about understanding the power system.
The
Marine Electrical and Electronics Bible
Chapter 2 -Batteries
This is the contents listing for batteries and it is extensive and detailed. Refer and read this chapter for in depth content.
About Batteries. Battery Rating Selection. Battery Rating Calculations. Sailing Load Calculation. Battery Ratings. Deep Cycle Batteries. Starting Batteries. Battery Technologies. Lead Acid Batteries. Battery Electrolyte. Battery Water. Battery Plate Sulfation. Battery Commissioning. Battery Voltages and Installation. Battery Routine Testing. Battery Additives. Low Maintenance Batteries. Gel Cell Batteries. Optima Batteries. Gel Cell Charging. AGM Batteries. Optima Batteries. Lithium-Ion Batteries. Lithium-Ion Battery Charging. Lithium-Ion Battery Installation. Lithium-Ion Battery Storage. Thermal Runaway. Battery Management Systems. Carbon Foam Batteries. Graphene Batteries. Lead Carbon Batteries. Alkaline Batteries. Battery Installation. Device Batteries. Buy a copy of the Marine Electrical and Electronics Bible.
Marine Electrical Courses Online Batteries
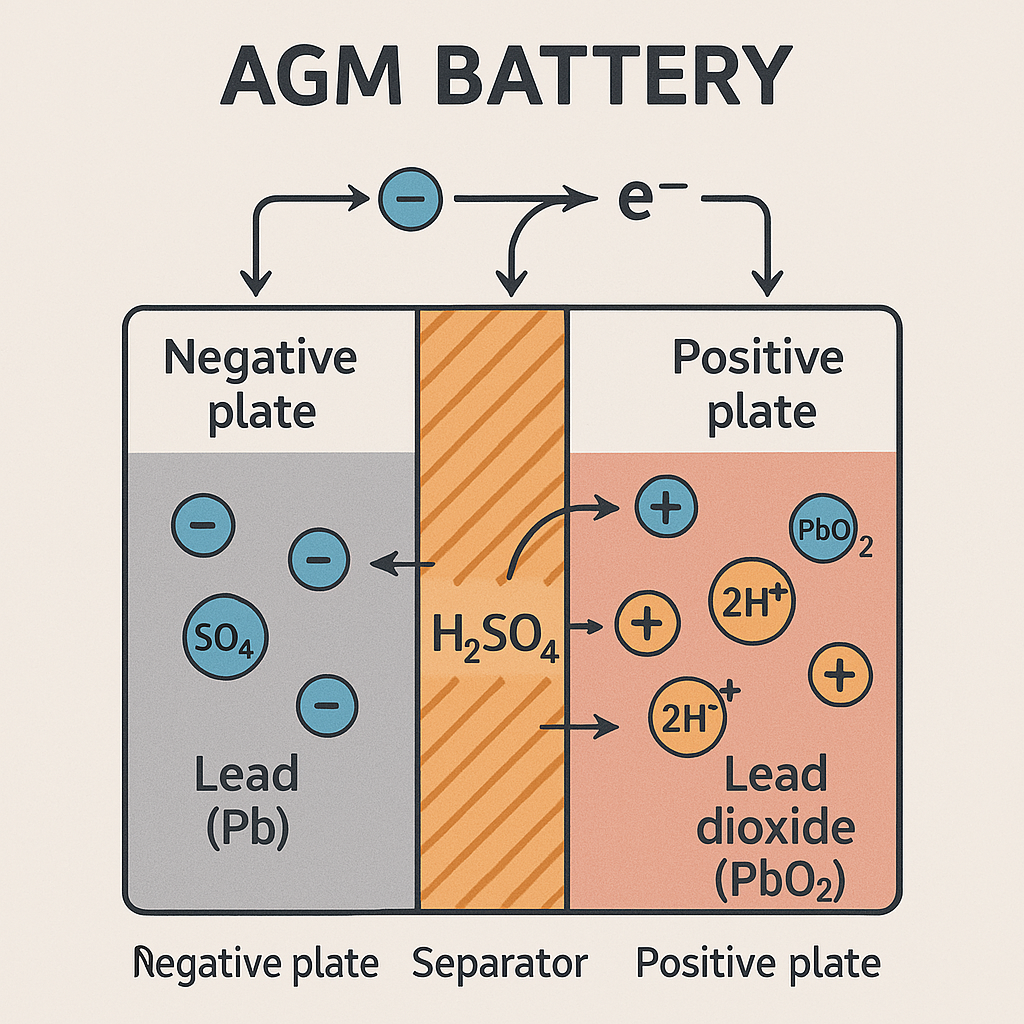
Back to basics. The fundamental theory of the battery is that a voltage is generated between two electrodes of dissimilar metal when they are immersed in an electrolyte. In the typical lead-acid flooded cell the generated voltage is nominally 2.1 volts. The typical 12-volt battery consists of 6 cells, which are internally connected in series to make up the battery. Each cell consists of the Positive plates, which are made from Lead Dioxide (PbO2), the Negative plates which are made from Sponge Lead (Pb), and they are immersed in the electrolyte made of Sulfuric Acid (H2SO4). Current flow and discharging of the battery occurs when an external load is connected across the positive and negative terminals, and a chemical reaction takes place between the two plate materials and the electrolyte. During this discharge reaction, the plates interact with the electrolyte to form lead sulfate and water, which dilutes the electrolyte, reducing the electrolyte density. As both plates become similar in composition, the cell loses the ability to generate a voltage. Re-charging of a cell reverses this reaction and the water decomposes to release hydrogen and oxygen, with the two plate materials being reconstituted to the original material.
When the plates are fully restored, and the electrolyte is returned to the nominal density the battery is completely recharged. Recommended densities are normally obtainable from battery manufacturers and can vary a little between batteries. In warm tropical locations it is common for batteries to have a reduced electrolyte density, which does not cause separator and grid deterioration as fast as temperate climate density electrolytes. Deionized and distilled water is the preferred for topping up cells however many use rainwater or straight out of the marina water faucet, which introduces impurities and degrades the plates.
Marine Electrical Courses Online -
Sulfation
Sulfation is the single greatest cause of flooded cell battery failure and the causes are relatively simple. During discharge, the chemical reaction causes both plates to convert to lead sulfate, and if recharging is not carried out promptly the lead sulfate starts to harden and crystallise. This is characterised by the formation of white crystals on the typically brown plates and is almost non- reversible. The immediate effect of sulfation is partial and permanent loss of capacity as the quantity of active material is reduced. Electrolyte density also partially decreases, as the chemical reaction during charging cannot be fully reversed. This sulfated material also introduces higher resistances within the cell and inhibits charging, and as the level of sulfated material increases, the cell's ability to retain a charge is reduced and the battery ultimately fails. The deep cycle battery has unfairly gained a bad reputation for sulfation, however the battery is not the cause, improper and incomplete charging is the real cause.
Marine Electrical Courses Online
Batteries - Lithium
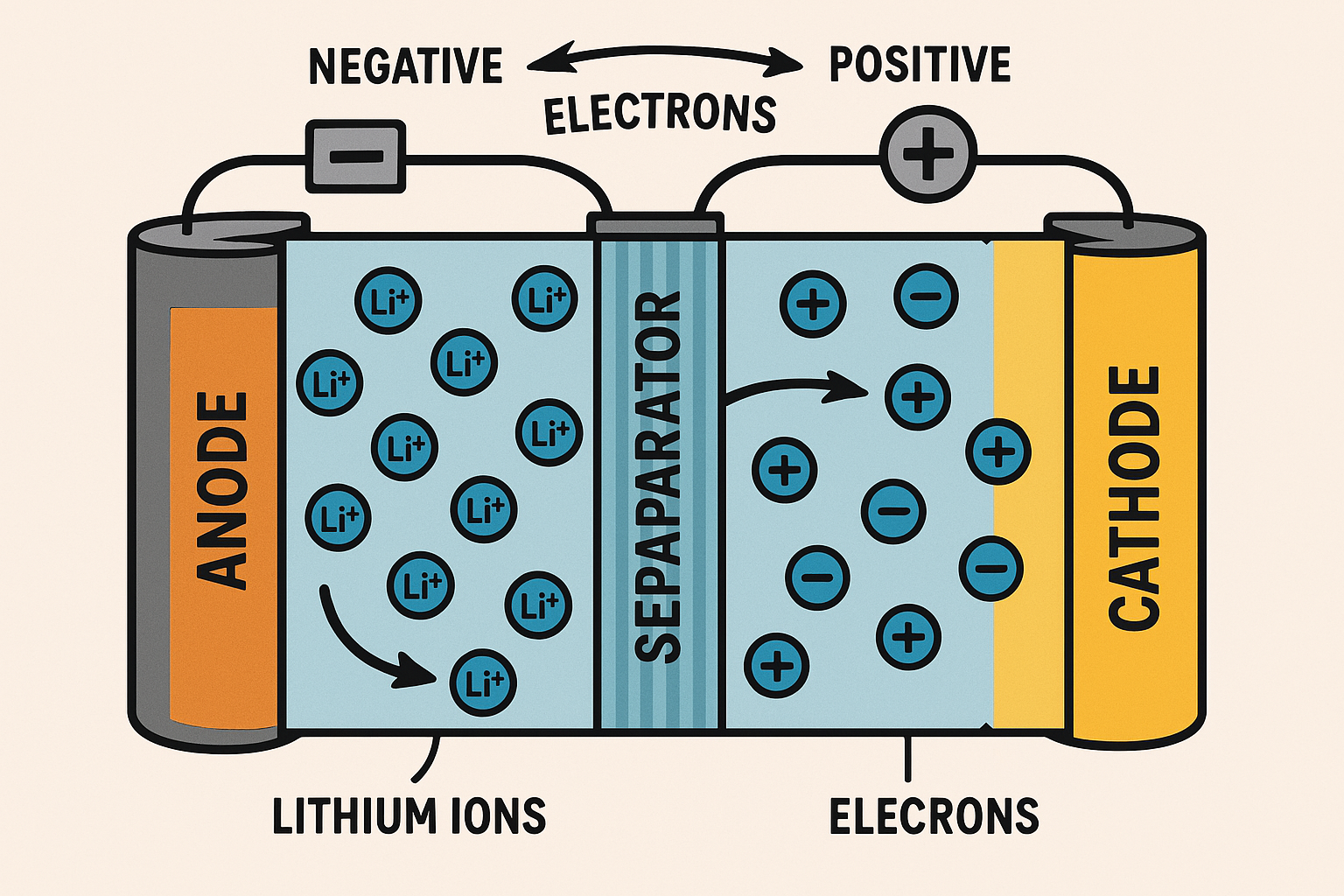
The lithium-ion battery consists of several key components that work together to store and release electrical energy efficiently. It is important to note that most boats install Lithium Iron Phosphate (LiFePO4) batteries which do not have the same issues as those used in electric vehicles or in portable devices. Lithium Ion battery information is here. These are the main parts of a Lithium ion battery:
- Cathode – The positive electrode, which determines the battery’s capacity and voltage. It is typically made of lithium-based compounds such as lithium cobalt oxide (LiCoO₂) or lithium iron phosphate (LiFePO₄).
- Anode – The negative electrode, usually made of graphite, which allows lithium ions to flow in and out during charging and discharging.
- Electrolyte – A liquid or gel-like substance that facilitates the movement of lithium ions between the anode and cathode. It’s often composed of lithium salts dissolved in organic solvents.
- Separator – A thin, porous membrane that prevents direct contact between the cathode and anode while allowing lithium ions to pass through.
- Current Collectors – Metal foils (typically aluminum for the cathode and copper for the anode) that collect and transfer the flow of electrons to the external circuit.
- Battery Casing – The protective enclosure that holds all the internal components together and prevents external damage.
When the battery is charging, lithium ions move from the cathode to the anode through the electrolyte. During discharge, they return to the cathode. Read more about Lithium Ion batteries here.
Marine Electrical Courses Online AGM Batteries
AGM batteries like Gel cells are also classed as Sealed Valve Regulated (SVR) batteries. The electrolyte is held within a very fine microporous (boron-silicate) glass matting that is placed between the plates, which absorbs and immobilizes the acid while still allowing rapid plate and acid interaction. Another term used for AGM batteries is starved electrolyte batteries, and this is because the glass matting is only 95% soaked in electrolyte. In a normal lead-acid battery, water loss will occur when it is electrically broken down into oxygen and hydrogen near the end of charging. In a battery during charging, oxygen will evolve at the positive plate at approximately 75% of full charge level, and Hydrogen evolves at the negative plate at approximately 90% of full charge. In normal batteries, the evolved gases disperse to atmosphere, resulting in electrolyte loss and periodic water replacement, and these are the bubbles seen in the cells during charging.
Marine Electrical Courses Online AGM Batteries
During charging the current causes decomposition of the water, and oxygen is evolves on the positive plate. The oxygen then migrates through the unfilled pores of the separator matting to react with the negative plate and form lead oxide, lead sulfate and water. The charge current reduces and does not generate hydrogen. The low maintenance recombinational battery has different characteristics. The plates and separators are held under pressure. During charging, the evolved oxygen is only able to move through the separator pores from positive to negative, reacting with the lead plate to recombine. The negative plate charge is then effectively maintained below 90% so inhibiting hydrogen generation. They emit less than 2% hydrogen gas during severe overcharge (4.1% is flammable level). The operational principle is called the recombinant gas absorbed electrolyte, as the generated gases recombine within the battery and significantly reduce hydrogen emissions. Marine Electrical Courses Online Batteries is a big subject.